Introduction
The multibillion dollar pet food industry has recently seen the addition of a new dietary therapy that promises to promote “thyroid health” in a can, or if you prefer, a bag. Despite the multimillion dollar advertising campaign waged by the Colgate-Palmolive subsidiary Hill’s Pet Nutrition that promotes an iodine deficient diet as a means of achieving “Thyroid Health”, thoughtful evaluation of their product yields serious concerns. To follow along as Dr. Peterson blogs about Hill’s y/d diet, click here.
Thyroid anatomy and physiology
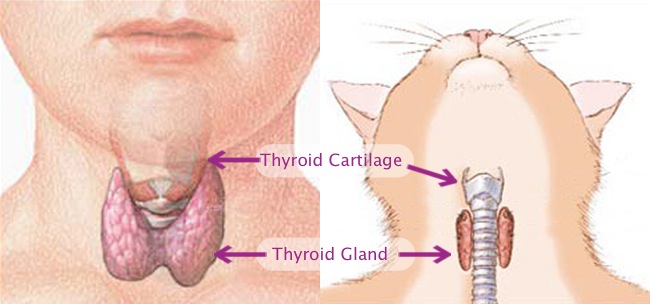
The thyroid is a small bi-lobed gland located in the cervical region (i.e., neck) in all mammals. The thyroid gland gets its name from the adjacent thyroid cartilage, often referred to as the “Adam’s Apple”, which is the largest cartilage of the larynx or voice box. The thyroid cartilage resembles an ancient Greek army shield called a thyreos which, like the cartilage, also had a notch at the top for the soldier’s chin [1] (Figure 1). The thyroid is one of the body’s endocrine glands. Endocrine glands get their name from their behavior of secreting hormones into (i.e., “endo”) the blood stream. The hormones produced by the endocrine glands circulate within the bloodstream throughout the body to regulate various bodily functions.
Figure 1. Illustration of the human and feline cervical regions with emphasis on the thyroid gland, a
small bi-lobed gland located in the neck in all mammals that gets its name from the adjacent thyroid
cartilage. In cats the central isthmus that connects the two lobes is often vestigial resulting in the presence
of two anatomically independent lobes.
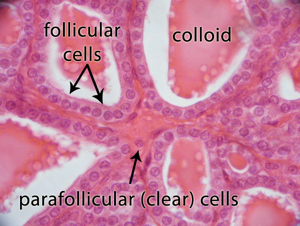
The thyroid gland consists of two cell types that differ based on their location and function (Figure 2). The majority of the cells within the thyroid gland are called follicular cells because they form numerous, microscopic, spherical structures called follicles. The follicular cells are responsible for the production of the thyroid hormones T3 (or triiodothyronine) and T4 (or thyroxine). The minority of cells in the thyroid are called parafollicular cells because they do not participate in the formation of thyroid follicles. The parafollicular cells produce a hormone called calcitonin that plays a relatively small role in calcium balance in the body [1].
Figure 2. Microscopic image of the thyroid gland which reveals the spherical
arrangement of the normal thyroid follicular cells surrounding the proteinaceous fluid,
called the colloid that contains the precursor to the thyroid hormones T3 and T4 called
thyroglobulin. The parafollicular cells that produce calcitonin are also evident in this image.
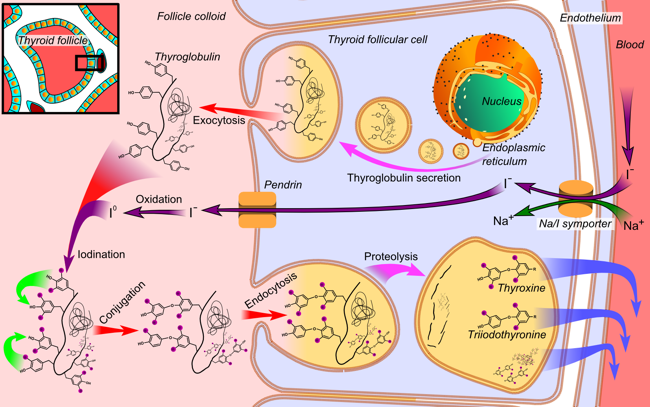
The follicles formed by the follicular cells surround a central cavity containing a material called the colloid which is rich in a hormone precursor, produced by the follicular cells, called thyroglobulin. One of the key steps in the production of thyroid hormones is the incorporation of iodine (i.e., iodination) into the hormone precursor thyroglobulin. This process necessitates the active uptake of iodide by the follicular cells of the thyroid (Figure 3). This utilization of iodide by the thyroid follicular cells is unique, as no other cells in the mammalian/vertebrate body require iodide. Ultimately thyroglobulin is broken down within the thyroid cells into the physiologically active thyroid hormones called thyroxine (i.e., T4) and triiodothyronine (i.e., T3) and then released into the blood stream. These thyroid hormones are ultimately responsible for setting the metabolic rate of the cells in the remainder of the body [2].
Figure 3. Illustration of a single thyroid follicular cell demonstrating the processes utilized to produce
thyroid hormone. Thyroglobulin is synthesized in the endoplasmic reticulum and follows the secretory pathway
to enter the colloid in the lumen of the thyroid follicle by exocytosis. Meanwhile, a sodium-iodide (Na/I) symporter
pumps iodide (I-) actively into the cell. This iodide enters the follicular lumen from the cytoplasm by the transporter pendrin,
in a passive manner. In the colloid, iodide (I-) is oxidized to iodine (I0) by an enzyme called thyroid peroxidase. Iodine (I0) is very
reactive and iodinates the thyroglobulin at tyrosyl residues in its protein chain. In conjugation, adjacent tyrosyl residues are paired together. The entire complex re-enters the follicular cell by endocytosis. Proteolysis by various proteases liberates thyroxine
and triiodothyronine molecules, which enters the blood.
Hyperthyroidism
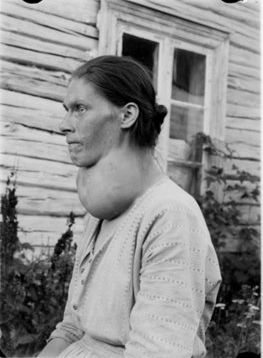
Hyperthyroidism is the clinical condition in which the thyroid gland produces excessive amounts of the thyroid hormones T3 and T4. Hyperthyroidism occurs in different species as the result of different mechanisms. In people, hyperthyroidism is relatively common and usually the result of an autoimmune disease called Graves’ disease named after the physician, Dr. Robert J. Graves. Graves’ disease causes a diffuse enlargement, or goiter, of the entire thyroid gland [3]. In dogs, hyperthyroidism is a relatively uncommon disease that is usually caused by a thyroid carcinoma (i.e., cancer of the thyroid gland)[4].
Figure 4. Hyperthyroid cat presented for radioiodine therapy.
Note the severe weight loss and muscle wasting.
In cats, hyperthyroidism is almost always caused by one or more small benign tumors of the thyroid gland called adenomas [5]. These thyroid adenomas function autonomously and hence produce excessive amounts of thyroid hormones. The disease that causes hyperthyroidism in cats is similar to Plummer’s disease (named after the physician Dr. Henry S. Plummer) in people that is also caused by autonomously functioning thyroid adenoma(s)[6]. The clinical signs of hyperthyroidism are the same in all species and reflect the stimulatory nature of the thyroid hormones T3 & T4 on the cells of the rest of the body. Excessive thyroid hormone levels lead to an increased metabolic rate that if unchecked, ultimately proves life limiting. (Figure 4)
Feline hyperthyroidism is currently the most commonly diagnosed endocrine disease of the cat. Since its initial discovery in 1979, the diagnosis of hyperthyroidism in cats has continued to increase, reaching epidemic proportions. Our incomplete understanding of the cause or causes of hyperthyroidism in cats has precluded effective prevention efforts. Since its recognition in 1979, a major suspect of the cause for the thyroid adenomas that lead to hyperthyroidism in cats has been commercially produced cat foods. Despite the epidemic proportions that hyperthyroidism has reached in cats since the late 1970’s, little has been written about the nutritional needs of the hyperthyroid cat. With the introduction of Colgate-Palmolive’s (i.e., Hills’ Pet Nutrition, Inc.) iodine deficient diet y/d the question of what to feed a hyperthyroid cat has once again surfaced [7]. The concept behind Hills’ iodine deficient diet y/d is that a diet deficient in iodine content will limit the ability of the thyroid follicular cells to produce the thyroid hormones T3 and T4. Because the production of thyroid hormones requires the incorporation of iodine into the hormone precursor thyroglobulin, iodine deficiency will lead to the reduced production of thyroid hormones.
History of iodine deficiency in man
The concept that iodine deficiency leads to the reduced production of thyroid hormones is well established. Indeed the history of iodine deficiency dates back to the ancient Greeks that recognized the value in treating patients with goiters (thyroid enlargements) using marine sponges. The Greeks however did not know that the thyroid enlargement they witnessed was due to the hypertrophy of normal thyroid cells in response to chronic iodine deficiency. Nor did they realize that marine sponges are an excellent source of iodine that will resolve the patient’s iodine deficiency and reverse the goiter [8].(Figure 5)
Figure 5. Large goiter (thyroid enlargement) due to iodine deficiency.
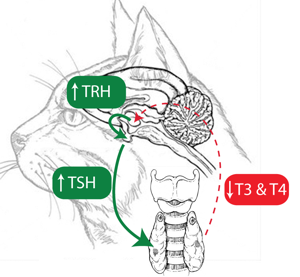
Iodine deficiency in patients with normal thyroid function results in the reduced production of thyroid hormones. This initially subclinical hypothyroidism stimulates the hypothalamus in the brain to increase production of thyrotropin releasing hormone (TRH) which in turn stimulates the increased production and release of thyroid stimulating hormone (TSH) from the pituitary gland.
The effect of increased TSH production by the pituitary is stimulation of the thyroid cells to grow and produce thyroid hormones. The chronic increase in TSH levels in the blood that accompanies iodine deficiency ultimately leads to the hypertrophy of the normal thyroid gland causing a goiter (see figure above) [9].
Iodine deficiency is an important human health problem throughout much of the world. Most of the earth’s iodine is found in oceans, and iodine content in the soil varies with region. The older an exposed soil surface, the more likely the iodine has been leached away by erosion. Mountainous regions, such as the Himalayas, the Andes, and the Alps, and flooded river valleys, such as the Ganges, are among the most severely iodine-deficient areas in the world. One fifth of the world’s human population (1 billion people) have diets that are deficient in iodine. People in these areas commonly have thyroid enlargement (goiter), and often thyroid nodules as well. In the United States, dietary iodine deficiency was essentially eliminated early in the last century with the addition of iodine to salt and its presence in milk and bread products [8].
Potential role of iodine deficiency in the etiology of feline hyperthyroidism
Hyperthyroidism was first diagnosed in cats in 1979, and its diagnosis has steadily increased ever since. The average age of cats diagnosed with hyperthyroidism was, and has remained 13 years of age. As a result early investigations into the cause of hyperthyroidism in cats looked at environmental changes that began during the 1960’s. Certainly one of the more significant of these changes was the introduction of commercially produced cat foods. Prior to the 1960’s most cats ate foods they were evolutionarily well adapted to, including small rodents, reptiles and birds. With the introduction of commercial cat foods in the 1960’s, the diet of cats dramatically changed to include large amounts of previously foreign foods including numerous plants, especially corn and soy. Since 1979 numerous articles have investigated the link between commercial cat foods and the epidemic of hyperthyroidism in cats. To date every epidemiologic study investigating hyperthyroidism in cats has found that consumption of commercial cat foods is a risk factor for developing the disease [10-17]. Both canned and dry cat foods have been implicated as factors in the development of hyperthyroidism in cats [12, 13, 18].
Figure 6. Decreased circulating T3 and T4 levels that occur in iodine
deficiency reduce feedback suppression and lead to increases in TRH release
from the hypothalamus which leads to increased TSH release from the pituitary.
Chronically increased TSH levels result in increased thyroid size i.e., goiter.
Toxic nodular goiter, called Plumber’s disease in man, is the human equivalent to hyperthyroidism in cats [5]. Human patients with Plumber’s disease, like cats with hyperthyroidism, have one or more, small, benign autonomously functional thyroid tumors called adenomas. Plumber’s disease is the most common cause of hyperthyroidism in the elderly. And Plumber’s disease, like hyperthyroidism in cats, occurs more commonly in females than in males [6, 13, 14, 16, 17]. The etiology of Plumber’s disease includes iodine deficiency and consumption of or exposure to goitrogens in food, water and the environment [19-22]. Goitrogens are substances that suppress the function of the thyroid gland by interfering with iodine uptake, which can, as a result, cause an enlargement of the thyroid, i.e., a goiter. The numerous similarities between Plumber’s disease and the adenomatous thyroid disease that causes hyperthyroidism in cats has lead many to consider the role of iodine deficiency in the development of hyperthyroidism in our aged cat population [23, 24]. In a recent case-control study, cats consuming commercial foods without iodine supplementation, according to listed ingredients, were more than four times as likely to develop hyperthyroidism compared with cats that ate iodine-supplemented foods [17].
Hill’s new iodine deficient diet y/d
It is easy to understand how Hill’s new iodine deficient diet y/d works to lower the circulating thyroid hormone levels in hyperthyroid cats. By starving the follicular cells of the thyroid for the iodine they need to make the thyroid hormones T3 and T4, any iodine deficient diet will lower circulating levels of the these hormones. There are however, several questions about this diet that remain.
Is it safe to feed an iodine deficient diet to hyperthyroid cats?
In light of our increasing awareness that iodine deficiency likely plays a role in the development of hyperthyroidism in cats, how advisable is it to intentionally feed a dramatically iodine deficient diet to cats that have already developed benign tumors of their thyroid? Will ongoing, severe iodine deficiency accelerate the growth of these benign tumors? Will severe ongoing iodine deficiency accelerate the progression from benign thyroid adenoma to malignant thyroid carcinoma [25]? Currently these questions remain to be answered. Minimally, we know that this diet does nothing to prevent the continued growth of the tumors responsible for hyperthyroidism in cats. Hill’s y/d includes at least two of the dietary factors that appear to contribute to the development of hyperthyroidism including overt iodine deficiency and soy isoflavins that act as goitrogens.
Is it safe to feed an iodine deficient diet to cats with normal thyroid function?
What about feeding y/d to cats without pre-existing thyroid disease? Many hyperthyroid cats live in multi-cat households. According to Hill’s web site, “Because iodine intake from other food sources — treats, another pet’s food, etc. — can compromise the effectiveness of low-iodine nutrition, it’s critical that you follow your veterinarian’s feeding instructions carefully and feed only y/d” [26]. Consumption of even small amounts of foods containing higher levels of iodine, including essentially all commercially available cat and dog foods and many human foods will negate the effect of y/d. This may pose a real problem to most multi-cat households in which the pet food and water resources are shared. While publicly acknowledging the potential risks of feeding an iodine deficient diet to cats without established thyroid disease, privately Hill’s representatives encourage veterinarians to recommend feeding y/d to all of the cats in multi-cat households as a means of overcoming this problem. They argue that supplementing the cats without hyperthyroidism with small amounts of other foods will overcome the deficiencies of the y/d product. In light of our current knowledge of pervasive iodine deficiencies in many commercially available cat foods, this logic seems flawed. Feeding y/d to every cat in a multi-cat household, just to ensure severe iodine deficiency in a single hyperthyroid cat seems sure to accomplish only one goal, namely increasing the sale of y/d.
In addition to the issues created by feeding an iodine deficient diet, Hill’s y/d has many other features that make it a poor choice for feeding cats as a species. Cats are after all obligate carnivores and Hill’s y/d is relatively low in protein, high in carbohydrates and relies on large quantities of plant based proteins, all of which make it a poor choice to feed to any cat [7, 27, 28]. The dry variety of y/d utilizes the cheap by-product filler “soybean mill run”. Soy has been shown to contain enzyme inhibitors that impeded normal protein digestion and soy is a known goitrogen suspected as a contributing factor in the development of hyperthyroidism in cats[18, 29-34].
How well does Hill’s iodine deficient diet y/d work to resolve hyperthyroid cat’s thyroid hormone elevations?
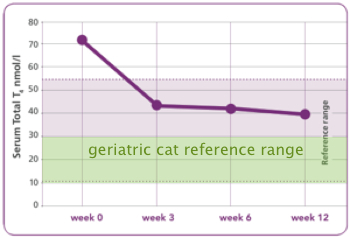
According to data provided by Hill’s Pet Nutrition, about 75% of hyperthyroid cats exclusively eating y/d will have normal serum total T4 concentrations by 4 weeks on the diet. By 8 weeks, 90% of cats have a serum T4 level; by 12 weeks, almost all cats should have values within the reference range [26].
It is well established, however, that geriatric cats normally have total T4 levels in the lower half of the reference range that was utilized in Hill’s studies [35]. In Hill’s own studies, hyperthyroid cats fed exclusively y/d did not routinely achieve these levels, even after 12 weeks on the diet. Preliminary evaluation suggests that this therapy appears to be more effective in cats with mild to moderate elevations of T4 and is not as effective in cats with severe hyperthyroidism. Furthermore, as an iodine deficient diet does not prevent, and may even encourage growth of the thyroid adenomas responsible for hyperthyroidism in cats, use of an iodine deficient diet appears unlikely to successfully control the thyroid hormone elevations over time.
Figure 7. Graph showing total T4 levels in hyperthyroid cats being fed y/d
exclusively for 12 weeks. Notice that T4 levels never reach the
ideal therapeutic range of 10-30 nmol/l (= 0.8-2.5 µg/dl).
(Graph from Hill’s Pet Nutrition, modified to reveal geriatric cat reference range.)
As the thyroid adenoma(s) responsible for hyperthyroidism continue to grow, the number of autonomously functional thyroid cells increases and their combined efficiency for extracting iodine from the blood stream with which to make thyroid hormones increases.
Conclusions
There is little doubt that feeding Hill’s new iodine deficient diet y/d has the potential to lower circulating thyroid hormone levels in hyperthyroid cats. The real question is whether a severely iodine deficient diet is the way to achieve “Thyroid Health” as suggested by the recent Hill’s marketing blitz. With what we know about the potential causes of hyperthyroidism in cats combined with the basic nutritional needs of the feline species it appears that a low protein, high carbohydrate, plant based, iodine deficient cat food is the epitome of what some have called “tunnel vision” nutrition. Indeed the more I learn about the new diet from Hill’s Pet Nutrition the more I find myself asking the question…
References
1. Capen C.C., Anatomy – Comparative Anatomy and Physiology, in Werner and Ingbar’s the Thyroid, a Fundamental and Clinical Text, L.E. Braverman and R.D. Utiger, Editors. 1996, Lippincott-Raven: Philadelphia. p. 19-37.
2. Taurog A., Hormone Synthesis – Hormone Synthesis: Thyroid Iodine Metabolism, in Werner and Ingbar’s the Thyroid, a Fundamental and Clinical Text, L.E. Braverman and R.D. Utiger, Editors. 1996, Lippincott-Raven: Philadelphia. p. 47-80.
3. Davies T.F., Graves’ Disease – the Pathogenesis of Graves’ Disease, in Werner and Ingbar’s the Thyroid, a Fundamental and Clinical Text, L.E. Braverman and R.D. Utiger, Editors. 1996, Lippincott-Raven: Philadelphia. p. 525-535.
4. Scarlett J.M., Epidemiology of Thyroid Diseases of Dogs and Cats. Vet Clin North Am Small Anim Pract, 1994. 24(3): p. 477-86.
5. Peterson M.E. and Becker D.V. Spontaneous Hyperthyroidism in the Cat: An Animal Model for Toxic Nodular Goiter in Scientific Proceedings Proceedings of the 59th Annual Meeting of the American Thyroid Association. 1983: p. T-31.
6. Hay I.D. and Morris J.C., Toxic Adenoma and Toxic Multinodular Goiter, in Werner and Ingbar’s the Thyroid, a Fundamental and Clinical Text, L.E. Braverman and R.D. Utiger, Editors. 1996, Lippincott-Raven: Philadelphia. p. 566-572.
7. Peterson M.E. The Best Diet to Feed Hyperthyroid Cats. Insights into Veterinary Endocrinology 2011.
8. Zimmermann M.B., Research on Iodine Deficiency and Goiter in the 19th and Early 20th Centuries. The Journal of Nutrition, 2008. 138(11): p. 2060-3.
9. Stubner D., Gartner R., Greil W., et al., Hypertrophy and Hyperplasia During Goitre Growth and Involution in Rats-Separate Bioeffects of TSH and Iodine. Acta Endocrinol (Copenh), 1987. 116(4): p. 537-48.
10. Scarlett Jm M., Jn, Feline Hyperthyroidism: A Descriptive and Case-Control Study. Prev Vet Med, 1988. 6: p. 295-309.
11. Kass P.H., Peterson M.E., Levy J., et al., Evaluation of Environmental, Nutritional, and Host Factors in Cats with Hyperthyroidism. J Vet Intern Med, 1999. 13(4): p. 323-9.
12. Martin K.M., Rossing M.A., Ryland L.M., et al., Evaluation of Dietary and Environmental Risk Factors for Hyperthyroidism in Cats. J Am Vet Med Assoc, 2000. 217(6): p. 853-6.
13. Edinboro C.H., Scott-Moncrieff J.C., Janovitz E., et al., Multivariate Analysis of Risk Factors for Feline Hyperthyroidism in New Zealand. N Z Vet J, 2005. 53(1): p. 53-8.
15. Wakeling J., Everard A., Brodbelt D., et al., Risk Factors for Feline Hyperthyroidism in the UK. J Small Anim Pract, 2009. 50(8): p. 406-14.
16. Edinboro C.H., Scott-Moncrieff J.C. and Glickman L.T., Environmental Risk Factors for Feline Hyperthyroidism: Pet Cats as Potential Sentinels for Public Health. Thyroid, 2004. 14: p. 759.
17. Edinboro C.H., Scott-Moncrieff J.C. and Glickman L.T., Review of Iodine Recommendations for Commercial Cat Foods and Potential Impacts of Proposed Changes. Thyroid, 2004. 14: p. 722.
18. Court M.H. and Freeman L.M., Identification and Concentration of Soy Isoflavones in Commercial Cat Foods. Am J Vet Res, 2002. 63(2): p. 181-5.
19. Braverman L.E., Iodine and the Thyroid: 33 Years of Study. Thyroid: Official Journal of the American Thyroid Association, 1994. 4(3): p. 351-6.
20. Braverman L.E. and Utiger R.D., Introduction to Thyrotoxicosis, in Werner and Ingbar’s the Thyroid, a Fundamental and Clinical Text, L.E. Braverman and R.D. Utiger, Editors. 1996, Lippincott-Raven: Philadelphia. p. 522-524.
21. Laurberg P., Pedersen K.M., Vestergaard H., et al., High Incidence of Multinodular Toxic Goitre in the Elderly Population in a Low Iodine Intake Area Vs. High Incidence of Graves’ Disease in the Young in a High Iodine Intake Area: Comparative Surveys of Thyrotoxicosis Epidemiology in East-Jutland Denmark and Iceland. Journal of Internal Medicine, 1991. 229(5): p. 415-20.
22. Gaitan E., Goitrogens in Food and Water. Annual Review of Nutrition, 1990. 10: p. 21-39.
23. Edinboro C.H., Scott-Moncrieff J.C. and Glickman L.T., Feline Hyperthyroidism: Potential Relationship with Iodine Supplement Requirements of Commercial Cat Foods. J Feline Med Surg, 2010. 12(9): p. 672-9.
24. Ranz D., Tetrick M., Opitz B., et al., Estimation of Iodine Status in Cats. J Nutr, 2002. 132(6 Suppl 2): p. 1751S-3S.
25. Hibbert A., Gruffydd-Jones T., Barrett E.L., et al., Feline Thyroid Carcinoma: Diagnosis and Response to High-Dose Radioactive Iodine Treatment. J Feline Med Surg, 2009. 11(2): p. 116-24.
26. Hills Pet Nutrition. Prescription Diet Y/D Feline Thyroid Health. Prescription Diet y/d Feline Thyroid Health 2011 [cited 2011 2011]; Available from: http://www.hillspet.com/products/pd-feline-yd-dry.html.
27. Peterson M.E. Is Hill’s Y/D a Nutritious Diet for Hyperthyroid Cats? Insights into Veterinary Endocrinology 2011.
28. Peterson M.E. Is the Protein Content of Hill’s Y/D Too Low to Restore and Maintain Muscle Mass in Cats with Hyperthyroidism? Insights into Veterinary Endocrinology 2011.
29. Divi R.L., Chang H.C. and Doerge D.R., Anti-Thyroid Isoflavones from Soybean: Isolation, Characterization, and Mechanisms of Action. Biochem Pharmacol, 1997. 54(10): p. 1087-96.
30. Doerge D.R. and Chang H.C., Inactivation of Thyroid Peroxidase by Soy Isoflavones, in Vitro and in Vivo. J Chromatogr B Analyt Technol Biomed Life Sci, 2002. 777(1-2): p. 269-79.
31. Doerge D.R. and Sheehan D.M., Goitrogenic and Estrogenic Activity of Soy Isoflavones. Environ Health Perspect, 2002. 110 Suppl 3: p. 349-53.
32. Ikeda T., Nishikawa A., Imazawa T., et al., Dramatic Synergism between Excess Soybean Intake and Iodine Deficiency on the Development of Rat Thyroid Hyperplasia. Carcinogenesis, 2000. 21(4): p. 707-13.
33. Ikeda T., Nishikawa A., Son H.Y., et al., Synergistic Effects of High-Dose Soybean Intake with Iodine Deficiency, but Not Sulfadimethoxine or Phenobarbital, on Rat Thyroid Proliferation. Jpn J Cancer Res, 2001. 92(4): p. 390-5.
34. White H.L., Freeman L.M., Mahony O., et al., Effect of Dietary Soy on Serum Thyroid Hormone Concentrations in Healthy Adult Cats. Am J Vet Res, 2004. 65(5): p. 586-91.
35. Skinner N.D., Thyroid Hormone Levels in Cats: Colony Average and the Decrease with Age. J Nutr, 1998. 128(12 Suppl): p. 2636S-8S.